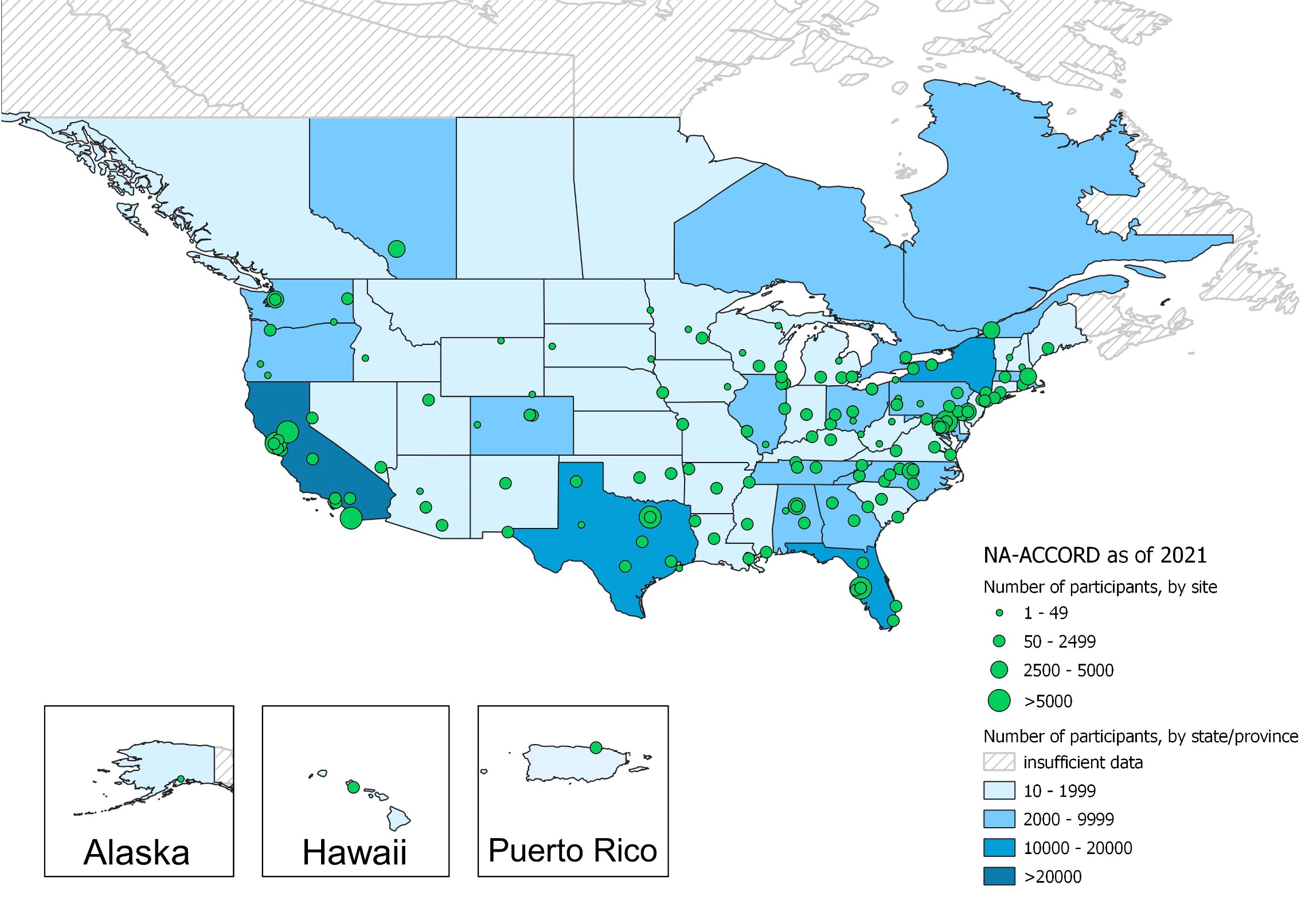
The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) began in 2006 as the North American regional representative of the International epidemiologic Databases to Evaluate AIDS (IeDEA). Comprised of over 20 collaborating cohorts, NA-ACCORD is widely representative of HIV care in the United States and Canada. Over 200 sites contribute data on over 190,000 HIV-infected participants.
NA-ACCORD has established a multi-disciplinary group of collaborators that span disciplines such as basic science, clinical research, epidemiology, data informatics and biostatistics. Collectively, our group is poised to answer the key questions in practice today. The NA-ACCORD has established a unique scientific platform to address clinical issues that are now of paramount importance in the modern treatment era.
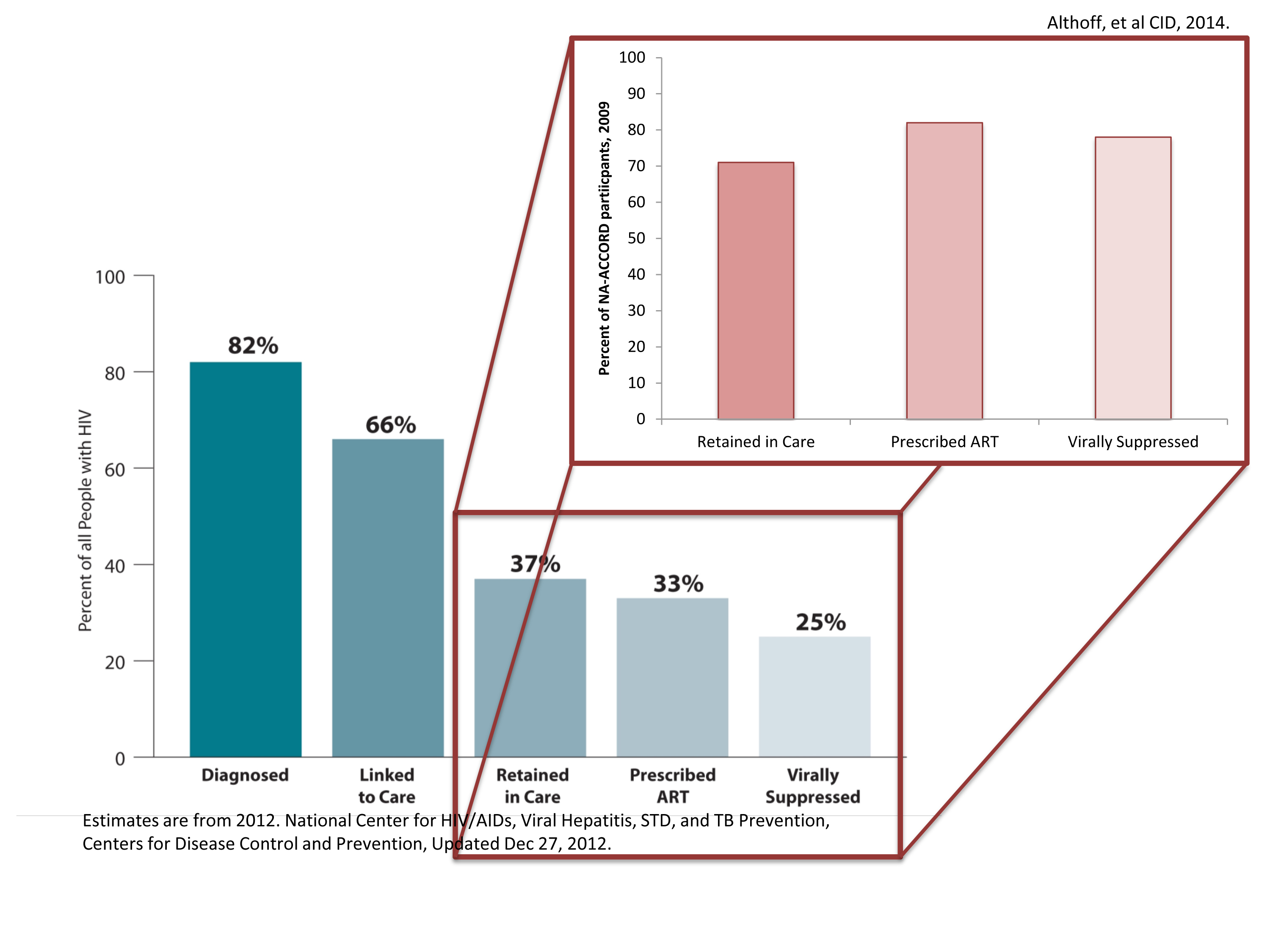
2012